Daily Newsletter February 16, 2012
Today's Topic: Redox and Living System
Energy harvesting will be our topic next week, so I wanted to spend a moment and talk about energy in biological systems. Energy is a word that is often thrown around in various disciplines, and we define it as the capacity to do work. This definition helps to simplify a complex issue, but when it comes down to it, it really doesn't help you to understand the bigger picture of how a complex set of reactions combine to form a living system.
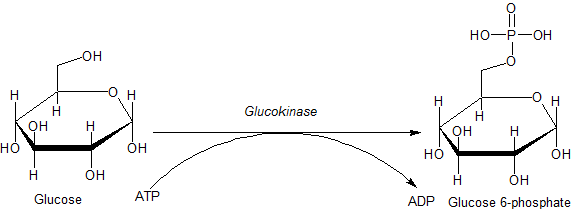
With ATP, you will
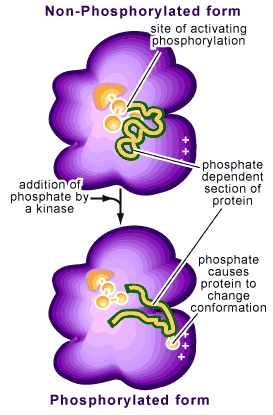
recall, the emphasis was shifted from seeing ATP as a batter that powered reactions. Instead, your focus was drawn to the Phosphate group, and the electrostatic effect it would have when added to a molecule or protein. Today in lecture we saw how a phosphate could act as an allosteric regulator, turning an enzyme on or off.
With energy harvesting, I again want you to shift your focus from a nebulous form of energy. This time, the focus will be on reducing potential. Central metabolism describes the oxidation of glucose, so what are we harvesting? Reducing potential. So what is reducing potential?
A simple definition is reducing potential describes the capacity of a compound to donate electrons. Chemistry has a strict definition involving measurements with electrodes, but for our purpose, the concept of donating electrons is what is important.
Remember the characteristics of life. You must maintain homeostasis, and this means repair. You have to build nucleic acids, lipids, carbohydrates and proteins. These biosynthetic pathways often require you to reduce substrates. To stay alive, you need a constant supply of electrons for reduction; you need reducing potential. If you don't get these high energy electrons for reduction, you die. We will also find that this reducing potential is needed for us to make ATP.
So, redox reactions become vital to our survival. Redox reactions are coupled Oxidation and Reduction reactions. One compound is oxidized as the next is reduced.
Remember, the molecules undergoing redox have to be close/touching. But in relative size, a cell is huge compared to a simple molecule. We may harvest electrons (oxidation) in one part of the cell, but use the harvested reducing potential in another part of the cell (reduction). Remember, you don't have free electrons; you can't throw electrons across the cytoplasm. So, how do we couple reactions that may be separated spatially? We use carriers!
Electron carriers, like nicotinamide adenine dinucleotide (NAD), accept electrons at the site of oxidation, and then donate electrons at the site of reduction. NAD is readily oxidized and reduced during metabolic reactions, and there is only a negligible loss of energy from the electrons carried (can we ever have NO loss of energy? why or why not?).
NAD is also classified as a coenzyme, meaning it must work with an enzyme to accept or donate electrons. NAD can not randomly go to a molecule and oxidize or reduce it; its action is regulated by enzymes. NAD then must bind to an enzyme that catalyzes an Oxidation, and NADH must bind to an enzyme that catalyzes a Reduction.
Daily Challenge: Action of nicotinamide adenine dinucleotide (NAD)
In the citric acid cycle is the following reaction:
In this reaction, malate is oxidized. How do you know? You know because NAD is reduced to NADH. Below is a ribbon model of the protein malate dehydrogenase. Within the protein, you will see two molecules of NAD represented as balls. NAD binds to the enzymes active site first, and then malate binds. Within the active site are both + and - amino acids.
Your task today, using the enzyme malate dehydrogenase, explain how enzymes work and explain how reducing potential is harvested from organic compounds.