Today you are going to look over a scientific article dealing with the evolution of metabolic processes. This is the first scientific article assigned, so I want to spend a little time going over how to read a scientific article.
One of the biggest struggles students have in science is reading scientific articles. These articles are dense, meaning they convey a great deal of information quickly. They are intended for experts, who have an understanding of the background, procedures and protocols. As a result, they are an obstacle when someone new to the field tries to figure out what the authors are saying in the paper.
Most people when they come to a scientific article start with the first line and then plow their way through. Novices become quickly overcome by the language, protocols and jargon, and just get frustrated. Experts rarely read the article from start to finish, but instead skip around. They use the break down of the article to focus their attention on what interests them.
All scientific articles start with an abstract. This is a summation of the paper, but be warned, this summation can be misleading. The purpose of the abstract is to provide a brief rundown of the paper so that people who are looking for an background or protocols can determine if the paper will be of use to them. WARNING: the abstract is for experts, not novices. More than one student has been burned by reading just the abstract and thinking they understood the paper.
While this may be heresy to some, skip the abstract. Don't read the abstract when you'rer assigned a paper. Remember, it is helpful to some one searching through articles. If you are assigned a paper by a teacher, skip the abstract. Instead, go right to the paper's introduction.
Scientific articles are generally broken down into section, with the most common being an introduction, methods, results and conclusion(discussion)*. The introduction holds the background for the paper, and generally includes why the author thinks the study is important. You can usually find the authors hypothesis and assumptions (research logic) here as well.
When you start reading the introduction, do not have a pencil or highlighter in hand. Just do a read through. If you don't understand something, skip it and go on. Just make it through once. On a note pad, write your first impressions. What stood out to you? Start a second read through, but this time have a highlighter, pen or pencil. Mark statements (does not have to be a full sentence) that you think are important. Write down any notes. Ultimately, your looking for a few things in the introduction:
- Why does the author think this topic is important?
- What has led to this current research?
- What is the author's hypothesis?
- New terms:
- Since your new to scientific papers, many of the terms will be new.
- To start, pick three that seem important to what the author is doing.
- Look them up and make a note of their definitions somewhere on your copy of the article. (a few words will do)
The METHODsection is one of the hardest to read for a novice, because the whole thing is filled with information on the exact procedures used. Unfortunately, this means you need to have background knowledge on how to do most of these procedures. But this is where we start to learn new techniques. On your first time through, just skim over the method section, but you need to come back to it (Just not immediately)
What to look for in the methods:
This ultimately depends on why you're reading the paper. Are you looking for a method? Are you trying to find an experimental protocol? Or are you trying to figure out how the author got their results? So the questions you ask could change depending upon your goals. The paper you have today has a non-standard arrangement, and the experimental protocol section is minimized (it is actually fleshed out in other sections). Generally though, you want to look to answer the following question:
- How did the author set up the experiment?
- Did they use models systems? (Did they go to a location or did they attempt to replicate the system?)
- What controld did they use?
- How many replicates did they have?
- What experimental methods did they use?
You should be able to trace the RESULTS back to particular methods used. A good author will provide you a story that leads from methods to results, and finally to a discussion about their conclusions. Results sections normally provide just the FACTS (evidence) that was generated from the methods. As the reader, you are looking to see if the results provide evidence supporting or refuting the authors hypothesis. Your also looking to see what the data says to you. Do the results tell you the same thing they "told" the author? i.e., the results will inform (be the foundation for) the author's conclusions. Based on the same evidence, do you reach the same conclusion? Why or why not? *NOTE: The article today combines results and discussions.
Again, you need to have a good background in the methods to understand and interpret results. So, your goal as a novice is to begin looking at the data to gain an understanding of what the data represents. This is about learning to read graphs and charts. Good authors will lead their reader through the data, but authors have different skills at conveying their data. For the paper today, take one graph, and see if you can figure out all that it is trying to convey. Read the results section, and find where the author discusses the graph. What are they trying to say? *NOTE: For today's paper, explain the results shown in Figure 1.
In the Discussion/Conclusion of the paper, the author attempts to tie together their results and present a logical case supporting their hypothesis. The emphasis here is on LOGICAL. How does the author support the hypothsis? What statements are made to demonstrate how the results support the hypothesis?
For the paper today, find a statement that you think shows where the author demonstrates the data supporting the hypothesis. Also, does the author address future directions for the research? Do they make specific claims?
Reference
Huber, C., Kraus, F., Hanzlik, M., Eisenreich, W. and Wächtershäuser, G. (2012), Elements of Metabolic Evolution. Chem. Eur. J., 18: 2063–2080. doi: 10.1002/chem.201102914Go to the GSU Library Homepage. Above the search box on the left, you will see a series of tabs. Click on the Journal tab. Type the word chemistry, and click GO. This will bring up a series of Chemistry Journals. You are looking for Chemistry : a European journal. Click on the Find It @ GSU button. You will need to sign-in if you are off campus; follow the sign-in proceedure.
You will then see a series of links that show the access GSU has to different versions. Click on the Full Text Online link. This will take you to the paper. You will have the option to open the paper as a PDF. This is the best way to get a copy to print or save. Open the paper and start to read and take notes.
Daily Challenge
Read the article listed above. In the forum, write about the article. The specific information you need to add includes:- Why does the author think this topic is important?
- What has led to this current research?
- What is the author's hypothesis?
- Three new terms.
- How did the author set up the experiment?
- New methods/protocolsa
- Analysis of Figure 1.
- How does the author link data to support hypothesis (conclusion)?
- One specific claim or future direction for research.
Optional Challenge
Review of Lecture on September 28, 2012.Link to Forum


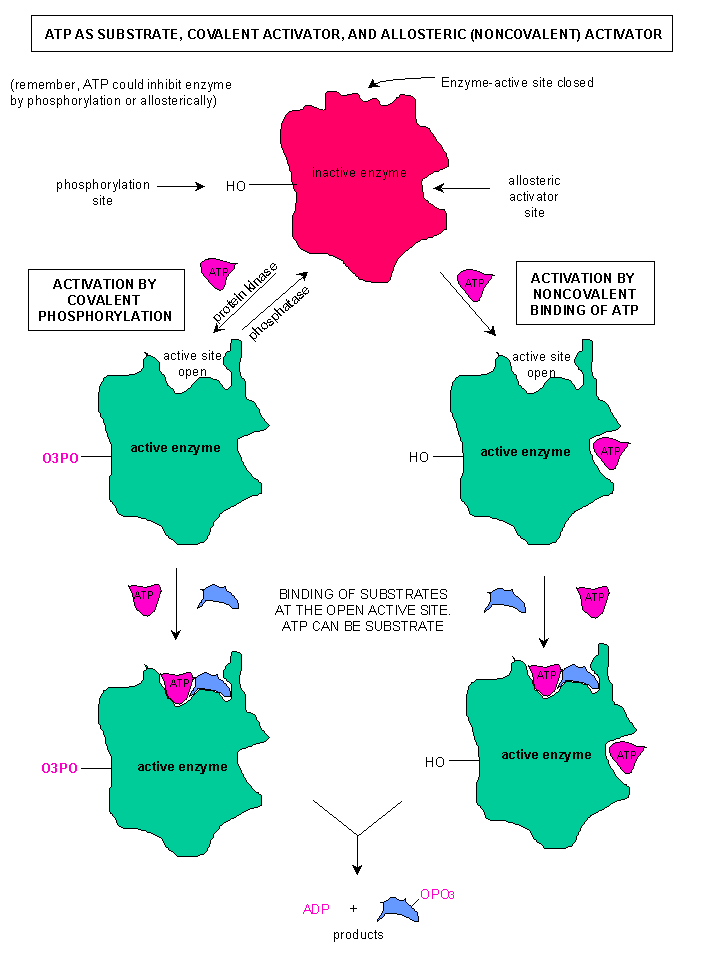
 The presence of AMP in the cell is a singal that we need to unlock our energy harvesting pathways, specifically, unlock phosphofructokinase. In this case, AMP acts as an activator to reverse the inhibitory effect of ATP. To the left is a diagram of phsophofructokinase. The area labeld "Regulatory Site" is the Allosteric site.
The presence of AMP in the cell is a singal that we need to unlock our energy harvesting pathways, specifically, unlock phosphofructokinase. In this case, AMP acts as an activator to reverse the inhibitory effect of ATP. To the left is a diagram of phsophofructokinase. The area labeld "Regulatory Site" is the Allosteric site. 


 Daily Newsletter
Daily Newsletter


 Daily Newsletter
Daily Newsletter You will recall this concept from chemistry, and it deals with reactions. On the right is an image of an Iron Thiocyanate reaction that was produced by the BBC. It shows the forward and reverse reactions. As you will remember, at a given set of conditions (such as a constant temperature and pressure) you can expect to see an equilibrium reached in which you will have some quantity of substrate and product. While the forward and reverse reactions will continue to occur, there will be a fairly constant quantity of substrate and product.
You will recall this concept from chemistry, and it deals with reactions. On the right is an image of an Iron Thiocyanate reaction that was produced by the BBC. It shows the forward and reverse reactions. As you will remember, at a given set of conditions (such as a constant temperature and pressure) you can expect to see an equilibrium reached in which you will have some quantity of substrate and product. While the forward and reverse reactions will continue to occur, there will be a fairly constant quantity of substrate and product.  Daily Newsletter
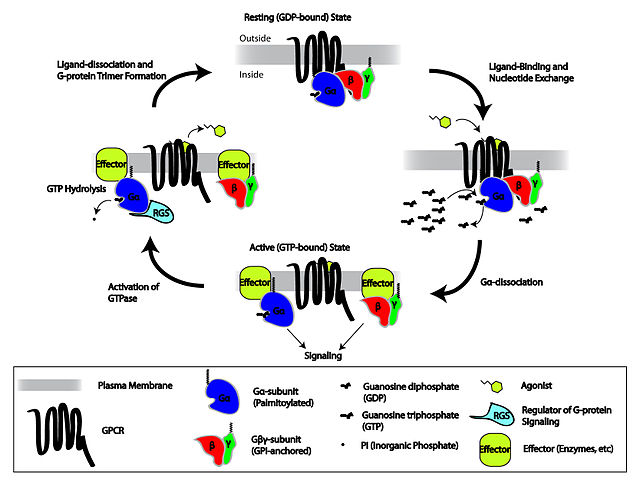
Daily Newsletter The system then uses a membrane component as the signal agent. The system also requires a second lipid component of the membrane known as diacylglycerol (DAG). To the right is a general schematic of the system from Wikipedia Commons.
The system then uses a membrane component as the signal agent. The system also requires a second lipid component of the membrane known as diacylglycerol (DAG). To the right is a general schematic of the system from Wikipedia Commons. Daily Newsletter
Daily Newsletter
 e Cyclase. This enzyme takes ATP (Adenosine Triphosphate) and removes two phosphates. The remaining phosphate binds back to the ribose sugar at the 3' carbon, creating a ring structure (cycle) between carbon 5' and 3'. This is known as cyclic AMP (cAMP). cAMP is a very common secondary messanger. It can be produced in bulk and can bind to a variety of enzymes. Many kinases (enzymes that add phosphates to other proteins, aka phosphorylation) are activated/deactivated by cAMP. So eukaryotic cells use this system to amplify the original signal to produce a large number of cAMP in order to affect protein activiation.
e Cyclase. This enzyme takes ATP (Adenosine Triphosphate) and removes two phosphates. The remaining phosphate binds back to the ribose sugar at the 3' carbon, creating a ring structure (cycle) between carbon 5' and 3'. This is known as cyclic AMP (cAMP). cAMP is a very common secondary messanger. It can be produced in bulk and can bind to a variety of enzymes. Many kinases (enzymes that add phosphates to other proteins, aka phosphorylation) are activated/deactivated by cAMP. So eukaryotic cells use this system to amplify the original signal to produce a large number of cAMP in order to affect protein activiation.
 Daily Newsletter
Daily Newsletter![Bensaccount at en.wikipedia, Author. “[Mu-opioid receptor (GPCR).png]” Wikimedia Commons. http://upload.wikimedia.org/wikipedia/commons/thumb/6/6e/Mu-opioid_receptor_%28GPCR%29.png/227px-Mu-opioid_receptor_%28GPCR%29.png (accessed September 19, 2012) μ-Opioid Receptor](http://upload.wikimedia.org/wikipedia/commons/thumb/6/6e/Mu-opioid_receptor_%28GPCR%29.png/227px-Mu-opioid_receptor_%28GPCR%29.png)
![Bensaccount at en.wikipedia, Author. “[Mu-opioid receptor.png]” Wikimedia Commons. http://upload.wikimedia.org/wikipedia/commons/thumb/b/b6/Mu-opioid_receptor.png/177px-Mu-opioid_receptor.png (accessed September 19, 2012) Active and inactive μ-opioid receptors.](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b6/Mu-opioid_receptor.png/177px-Mu-opioid_receptor.png)
 Daily Newsletter
Daily Newsletter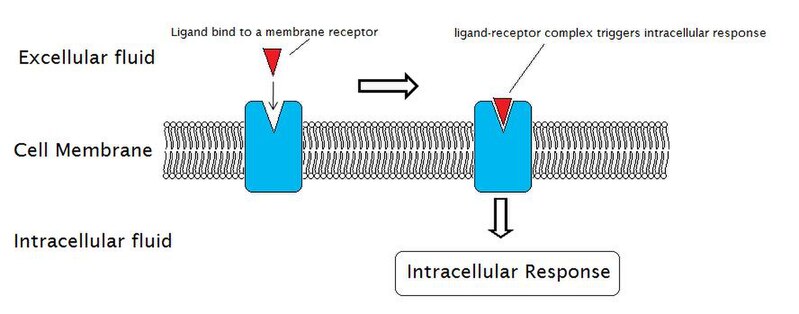


 Daily Newsletter
Daily Newsletter




 Daily Newsletter
Daily Newsletter



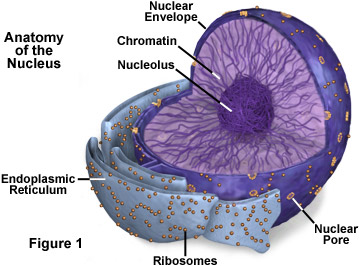
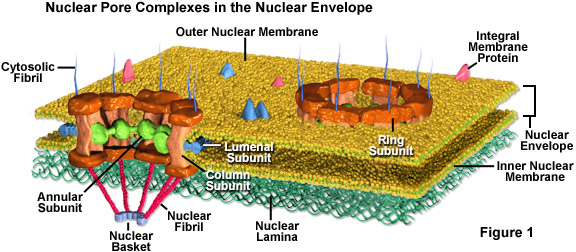

 Daily Newsletter
Daily Newsletter