Below are links to biology timelines created by students in Principles of Biology I (BIOL 2107)
Group 1
Group 2
Group 3
Group 4
Group 5
Group 6
Group 7
Group 8
Group 9
Group 10
Group 11
Group 12
This site archives newsletters dedicated to my open biology courses taught from Georgia State University in Atlanta, GA. The course focuses on the principles of cell and molecular biology. You are welcome to use the material, but please provide a link back to this blog.
Monday, October 12, 2015
Monday, May 18, 2015
Update: Summer 2015
Over the next few months, I am going to be turning the content of this blog into an eBook for my students. Once I'm done with the process, I'll post it here.
As part of the process, I will be moving all newsletters that were posted in my LMS over to this blog.
I will also be making some office mixes to add interactive content.
As part of the process, I will be moving all newsletters that were posted in my LMS over to this blog.
I will also be making some office mixes to add interactive content.
Thursday, January 15, 2015
Special Newsletter Gibbs Free Energy and Transition State Energy (Reposting)
Special Newsletter
Gibbs Free Energy and Transition State Energy
This goal of this newsletter is to help students navigate the complexity of Gibbs Free Energy (ΔG) and help avoid confusion about Gibbs Free Energy and Transition State Energy (Energy of Activation).
Gibbs Free Energy (ΔG) is a thermodynamics concept, and describes the thermodynamic potential of a system. It measures the "work" that can be obtained from a system, for us a chemical reaction, if that system is held at constant temperature and pressure. The goal is to determine whether a given reaction is spontaneous in the direction written, i.e., whether the reaction will proceed in the given direction without the input of energy.
Mathematically, Gibbs Free Energy is described as:
ΔG=ΔH−TΔS
Where:
Gibbs Free Energy (ΔG) is a thermodynamics concept, and describes the thermodynamic potential of a system. It measures the "work" that can be obtained from a system, for us a chemical reaction, if that system is held at constant temperature and pressure. The goal is to determine whether a given reaction is spontaneous in the direction written, i.e., whether the reaction will proceed in the given direction without the input of energy.
Mathematically, Gibbs Free Energy is described as:
ΔG=ΔH−TΔS
Where:
- ΔG The change in Free Energy
- ΔH The change in Enthalpy
- ΔS The change in Entropy
In biochemistry, the change in Enthalpy (ΔH) is the same as the change in internal energy. Remember that ΔG is calculated based on a set of given conditions: we are at constant temperature and pressure, and all variables have been taken into account (such as the solvent of the system). If you change anything, you change the system, and thus the ΔG.
Gibbs free energy is a measure ONLY of the difference in free energy of the products and reactants, and does not tell us about the rate of the reaction. It only tells us whether the reaction is Exergonic or Endergonic.
Gibbs free energy is a measure ONLY of the difference in free energy of the products and reactants, and does not tell us about the rate of the reaction. It only tells us whether the reaction is Exergonic or Endergonic.
- ΔG < 0 Exergonic: The reaction is considered spontaneous in the direction written.
- ΔG = 0 The system is in equilibrium
- ΔG >0 Endergonic: To carry out this reaction as written, there needs to be an addition of free energy (NOTE: this means that the reverse reaction is spontaneous).
Transition State Energy is not Gibbs Free Energy.
Gibbs Free Energy does not describe the path of transformation or the mechanism of transformation. It does not describe the rate of transformation. It only describes whether the reaction is spontaneous, non-spontaneous or at equilibrium.
The breakdown of the dissacharide sucrose to glucose and fructose has a ΔG of -5.5 kcal/mol. This is a spontaneous (Exergonic) reaction in terms of ΔG. Yet you can store sucrose in your kitchen and it remains sucrose. There is no spontaneous degradation into monosaccharides. Why?
Sucrose is a stable molecule. In order to force the breakdown (catabolism) of sucrose, we need to destabilize the molecule. This destabilization is the transition state of the reaction, and in a closed system, requires the input of energy. This is termed the Activation Energy of the reaction (you will also hear this described as the Transition State Energy). If we were to look at our breakdown of sucrose, we would see the following:
Sucrose ⇄ Transition State → Glucose + Fructose
The energy of the transition state is noted as either Ea or ΔG‡. These two expression are from different formula for calculating activation energy. ΔG‡ is used in a formula that relates activation energy to Gibbs Free Energy (the Eyring equation). In either case, the expression describe transition state energy (aka, activation energy).
In biochemistry, enzymes are used to reduce the activation energy (just like catalysts are used in chemistry). Catalysts and Enzymes reduce the activation energy ΔG‡; they do not alter Gibbs Free Energy (ΔG). This is a critical concept!
Transition state deals with the rate of the reaction. By lowering the activation energy (transition state energy), you increase the rate of the reaction. In essence, you are making the reaction more likely to happen. But the enzyme does not change Gibbs Free Energy.
 |
| Original Image from ChemWiki, http://chemwiki.ucdavis.edu/@api/deki/files/10017/fREE_eNERgY_cHART.jpg?size=bestfit&width=471&height=294&revision=1 |
Take home message:
Gibbs Free Energy (ΔG) describes whether a reaction is exergonic or endergonic. It does not describe rate, and neither enzymes or catalysts will alter ΔG.
Activation Energy (ΔG‡) does not alter ΔG; it does not determine whether a reaction is spontaneous or non-spontaneous. Activation energy does help determine the rate of the reaction.
Activation Energy (ΔG‡) does not alter ΔG; it does not determine whether a reaction is spontaneous or non-spontaneous. Activation energy does help determine the rate of the reaction.
Daily Newsletter September 24, 2014 Redox and Coupled Reactions (Reposting)
 Daily Newsletter
Daily Newsletter
September 24, 2014
Redox and Coupled Reactions
Energy harvesting will be our topic next week, so I wanted to spend a moment and talk about energy in biological systems. Energy is a word that is often thrown around in various disciplines, and most of us carry misconceptions about the world from colloquial (common) use of the word. Scientists define energy as the capacity to do work. This definition helps to simplify a complex issue, but it starts to get confused when we apply it to the complex sets of reactions that we see in living systems.
For example, as we saw yesterday, the work in phosphorylation occurs in creating the covalent bond between a phosphate group and a substrate, not the action of the substrate. With ATP, the emphasis was shifted from seeing ATP as a battery that powered reactions. Instead, your focus was drawn to the Phosphate group, and the electrostatic effect it would have when added to a molecule or protein.
Next week we will discuss Energy Harvesting. Like with ATP, I want you to focus your attention on a specific form of energy, instead of holding a nebulous concept. Today the focus will be on reducing potential. Central metabolism describes the oxidation of glucose, so what are we harvesting? Reducing potential. So what is reducing potential?
A simple definition is reducing potential describes the capacity of a compound to donate electrons. Chemistry has a strict definition involving measurements with electrodes, but for our purpose, the concept of donating electrons is what is important.
Remember the characteristics of life. You must maintain homeostasis, and this means repair. You have to build nucleic acids, lipids, carbohydrates and proteins. These biosynthetic pathways often require you to reduce substrates. To stay alive, you need a constant supply of electrons for reduction; you need reducing potential. If you don't get these high energy electrons for reduction, you die. We will also find that this reducing potential is needed for us to make ATP.
Redox reactions are vital to our survival. Redox reactions are coupled Oxidation and Reduction reactions. One compound is oxidized as the next is reduced.
For example, as we saw yesterday, the work in phosphorylation occurs in creating the covalent bond between a phosphate group and a substrate, not the action of the substrate. With ATP, the emphasis was shifted from seeing ATP as a battery that powered reactions. Instead, your focus was drawn to the Phosphate group, and the electrostatic effect it would have when added to a molecule or protein.
Next week we will discuss Energy Harvesting. Like with ATP, I want you to focus your attention on a specific form of energy, instead of holding a nebulous concept. Today the focus will be on reducing potential. Central metabolism describes the oxidation of glucose, so what are we harvesting? Reducing potential. So what is reducing potential?
A simple definition is reducing potential describes the capacity of a compound to donate electrons. Chemistry has a strict definition involving measurements with electrodes, but for our purpose, the concept of donating electrons is what is important.
Remember the characteristics of life. You must maintain homeostasis, and this means repair. You have to build nucleic acids, lipids, carbohydrates and proteins. These biosynthetic pathways often require you to reduce substrates. To stay alive, you need a constant supply of electrons for reduction; you need reducing potential. If you don't get these high energy electrons for reduction, you die. We will also find that this reducing potential is needed for us to make ATP.
Redox reactions are vital to our survival. Redox reactions are coupled Oxidation and Reduction reactions. One compound is oxidized as the next is reduced.
Remember, the molecules undergoing redox have to be close/touching. But in relative size, a cell is huge compared to a simple molecule. We may harvest electrons (oxidation) in one part of the cell, but use the harvested reducing potential in another part of the cell (reduction). Remember, you don't have free electrons; you can't throw electrons across the cytoplasm. So, how do we couple reactions that may be separated spatially? We use carriers!
Electron carriers, like nicotinamide adenine dinucleotide (NAD+), accept electrons at the site of oxidation, and then donate electrons at the site of reduction. NAD+ is readily oxidized and reduced during metabolic reactions, and there is only a negligible loss of energy from the electrons carried (can we ever have NO loss of energy? why or why not?).
NAD+ is also classified as a coenzyme, meaning it must work with an enzyme to accept or donate electrons. NAD+ can not randomly go to a molecule and oxidize or reduce it; its action is regulated by enzymes. NAD+ then must bind to an enzyme that catalyzes an Oxidation, and NADH must bind to an enzyme that catalyzes a Reduction.

Specifically, we couple the reactions. NAD+ has a place to bind into the enzyme, many times next to the substrate. The NAD+ can then capture the eletron pair that is released from the substrate. Additionally, one of the hydrogens will bind to the electron carrier. When we move to the next reaction, NADH will bind with an enzyme, again normally next to the substrate in question. The NADH can then donate the electrons (and hydrogens to the substrate. Enzymes thus help to couple these reactions. NAD+ will not just pick up an electron from any source, and NADH will not just donate electrons to any source. It must be mediated by enzymes.

Electron carriers, like nicotinamide adenine dinucleotide (NAD+), accept electrons at the site of oxidation, and then donate electrons at the site of reduction. NAD+ is readily oxidized and reduced during metabolic reactions, and there is only a negligible loss of energy from the electrons carried (can we ever have NO loss of energy? why or why not?).
NAD+ is also classified as a coenzyme, meaning it must work with an enzyme to accept or donate electrons. NAD+ can not randomly go to a molecule and oxidize or reduce it; its action is regulated by enzymes. NAD+ then must bind to an enzyme that catalyzes an Oxidation, and NADH must bind to an enzyme that catalyzes a Reduction.

Specifically, we couple the reactions. NAD+ has a place to bind into the enzyme, many times next to the substrate. The NAD+ can then capture the eletron pair that is released from the substrate. Additionally, one of the hydrogens will bind to the electron carrier. When we move to the next reaction, NADH will bind with an enzyme, again normally next to the substrate in question. The NADH can then donate the electrons (and hydrogens to the substrate. Enzymes thus help to couple these reactions. NAD+ will not just pick up an electron from any source, and NADH will not just donate electrons to any source. It must be mediated by enzymes.
Daily Challenge
This week you must complete all four forums to be eligible for the weekly summary and summative quiz.
Completion requires that you start a discussion, that your discussion is a minimum of 150 words on topics and at a collegiate level of writing, and that you reply to 3 of your fellow students.
Forum Closes: Septembe 25, 2014 at 11:55pm
Completion requires that you start a discussion, that your discussion is a minimum of 150 words on topics and at a collegiate level of writing, and that you reply to 3 of your fellow students.
Forum Closes: Septembe 25, 2014 at 11:55pm
Action of nicotinamide adenine dinucleotide (NAD)
In the citric acid cycle is the following reaction:
In the citric acid cycle is the following reaction:
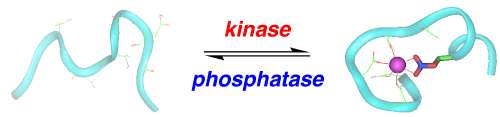
In this reaction, malate is oxidized. How do you know? You know because NAD is reduced to NADH. Below is a ribbon model of the protein malate dehydrogenase. Within the protein, you will see two molecules of NAD represented as balls. NAD binds to the enzymes active site first, and then malate binds. Within the active site are both + and - amino acids.
Your task today, using the enzyme malate dehydrogenase, explain how enzymes work and explain how reducing potential is harvested from organic compounds.
Your task today, using the enzyme malate dehydrogenase, explain how enzymes work and explain how reducing potential is harvested from organic compounds.
Daily Newsletter September 23, 2014 Adenosine Triphosphate (Reposting)
Daily Newsletter
September 23, 2014 Adenosine Triphosphate
You have most likely heard ATP referred to as the "energy currency" of the cell. In fact, your textbook uses this analogy: "Just as it is more effective, efficient, and convenient for you to trade money for a lunch than to trade your actual labor, it is useful for cells to have a single currency for transferring energy between different reactions and cell processes."
This is a lovely fiction that does not serve molecular biologists. It is a convenient expression, but it conveys a very serious misconception.
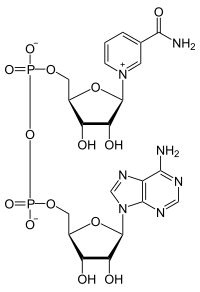
Nucleotide triphosphates (ATP, GTP, CTP, TTP and UTP) have their foundation in the nucleotide structure, with the addition of extra phosphate groups. Adenosine Triphosphate is the most prevalent nucleotide triphosphate, and is found as a cofactor in a number of enzymatic reactions. The picture below show the general structure of ATP.
NOTE: the nucleotide triphosphates use RIBOSE as the sugar. If the nucleotide triphosphate utilized deoxyribose, we add the letter "d" in front of the abbreviation; so dATP represents a deoxyribo-nuclotide. The only time the cell ultilizes dATP, dGTP, dCTP and dTTP is during the process of replication (DNA synthesis).
You have three phosphate groups, each with a negative charge, covalently bonded to each other. The phosphate groups naturally want to repel each other, but they are held together by one of the strongest bond types (covalent bonds). What does this mean? Molecular tension! But it must be noted that ATP is chemically stable. It does not spontaneously loose phosphates (if it did, you would also release heat). It takes enzymatic action to remove the phosphate (i.e., we have to break the covalent bond). When a phosphate is removed from ATP, it is generally attached to another molecular structure (enzymes, sugars, etc...). The exception to this will be in building nucleic acids.
This is a lovely fiction that does not serve molecular biologists. It is a convenient expression, but it conveys a very serious misconception.

Nucleotide triphosphates (ATP, GTP, CTP, TTP and UTP) have their foundation in the nucleotide structure, with the addition of extra phosphate groups. Adenosine Triphosphate is the most prevalent nucleotide triphosphate, and is found as a cofactor in a number of enzymatic reactions. The picture below show the general structure of ATP.
NOTE: the nucleotide triphosphates use RIBOSE as the sugar. If the nucleotide triphosphate utilized deoxyribose, we add the letter "d" in front of the abbreviation; so dATP represents a deoxyribo-nuclotide. The only time the cell ultilizes dATP, dGTP, dCTP and dTTP is during the process of replication (DNA synthesis).
You have three phosphate groups, each with a negative charge, covalently bonded to each other. The phosphate groups naturally want to repel each other, but they are held together by one of the strongest bond types (covalent bonds). What does this mean? Molecular tension! But it must be noted that ATP is chemically stable. It does not spontaneously loose phosphates (if it did, you would also release heat). It takes enzymatic action to remove the phosphate (i.e., we have to break the covalent bond). When a phosphate is removed from ATP, it is generally attached to another molecular structure (enzymes, sugars, etc...). The exception to this will be in building nucleic acids.
Chemist vs. Biologist
In yesterday's newsletter, a distinction was made between the perspective of chemists and biologists. If you look in most books that deal with biology, you will find the following euqation for ATP:
ATP + H2O → ADP + Pi ΔG˚ = −30.5 kJ/mol (−7.3 kcal/mol)
This is a look at ATP hydrolysis in isolation. Do you remember how ΔG is calculated? Look at the units. kJ (kilo-Joules) or kcal (kilocalories). We are basing this on an isolated reaction and measuring heat. When you look at the ΔG when there are metal ions present, you get a different number. You should also be familiar with ΔG, or Gibbs Free Energy, which is a measure of the amount of work that can be acheived by the energy release. A ΔG˚ = −30.5 kJ/mol is big, and implies that there is a great deal of energy released (the molecule can perform work).
Biologists though recognize that ATP is not in isolation. The intracellular fluid compartment (cytosol) contains ions, and more importantly metal ions, especially Mg2+ (causes major changes in hydrolysis ΔG). Inside of cells, the ΔG˚ of ATP hydrolysis is higher, approximately -50 kJ/mol (-12 kcal/mol). If we released this much energy every time ATP was hydrolyzed, the cell would boil! But, we use this energy for something else: binding the phosphate to another substrate (phosphorylation). When we phosphorylate a compound, we are building a covalent bond between the phosphate and the compound. This requires energy.1 About half the free energy is going to be used to make this covalent bond between the phosphate and the substrate. What happens with the rest? The second law of thermodynamics tells us that some of it is lost (this is an energy transfer after all...we broke one bond to build another), but the bond it self will hold some as well. Remember, chemical bonds are Potential Energy. When phosphate is removed, there will be another change in free energy (every reaction has a ΔG).
So what is the misconception with "energy currency"?
To answer this, we need to ask two other questions: why do we think of ATP as having High Energy Bonds and what is enery to a cell?
Why do we think of ATP in terms of High Energy Bonds? The answer is in the discussion above. The phosphoanhydride bonds (covalent bonds between the phosphate groups) have a high ΔG when they are hydrolyzed. This is why they have been called "high energy bonds". But in biology, the energy is used to immediately allow the phosphate group to bind to a new substrate (Phosphorylation). Put another way, the work that is done is in forming the covalent bond between the phosphate and the new substrate! The ATP is NOT a battery that energizes a curcuit.
ATP is always used in coupled reactions.
What is energy to a cell? Ask yourself, in building bonds in chemistry, what is the energy? If you want to change the energy state in a molecule, what do you do? Isn't it all about the electrons. Ultimately, the energy cells really use will be found with electrons, specifically through redox reactions. We will see that reducing potential is the energy the cells are harvesting and storing. This will be our discussion tomorrow.
Now we come to the big question: What does ATP do?
The concept of ATP as an "energy currency" comes from ATP turning on enzymes or assisting an enzyme during a "power steps" in a metabolic pathways. But ATP does not add energy; it just rearranges charge distribution around a molecule (electrochemistry). Remember, the phosphate group is negatively charged (-2).
When you add a phosphate group to a protein, you change the electrical signature around that portion of the protein (same will be true of other molecules as well). This includes the ability to form new hydrogen bonds, which can alter both secondary and tertiary structures. What will happen to the protein? It will change shape (conformational change). The work that ATP enables (and again this relates back to the free energy) is it's ability to cause conformational changes and induce molecular tension (instabilities).
Above is a general diagrapm showing a phosphorulation event. Notice how the phosphate group has interacted through van der Waals forces to change the tertiary structure of the protein.
The image below is a variant example of how ATP interacts with proteins. In this case, we see the the interaction of Myosin and Actin during muscle contraction. Focus your attention on the myosin molecule in blue. Notice that the "head" of the myosin changes conformation (shape) as ATP binds and is hydrolyzed. This is an important concept. When ATP binds, and hydrolysis occurs, a conformation change occurs. When ADP and Pi leave, the molecule resumes its "resting" conformation. In this case, it is the binding of the entire ATP molecule (with multiple negative charges) and subsequent hydrolysis which causes the conformational change. It still comes down to changing the electrical profile of the molecule (NOTE: there are more conformational changes occuring than are seen in this).
.
As we will see in upcoming weeks, this change of shape is critical to enzyme function. You have already encountered this once before, with the Sodium/Potassium ATPase.
The image below is a variant example of how ATP interacts with proteins. In this case, we see the the interaction of Myosin and Actin during muscle contraction. Focus your attention on the myosin molecule in blue. Notice that the "head" of the myosin changes conformation (shape) as ATP binds and is hydrolyzed. This is an important concept. When ATP binds, and hydrolysis occurs, a conformation change occurs. When ADP and Pi leave, the molecule resumes its "resting" conformation. In this case, it is the binding of the entire ATP molecule (with multiple negative charges) and subsequent hydrolysis which causes the conformational change. It still comes down to changing the electrical profile of the molecule (NOTE: there are more conformational changes occuring than are seen in this).

.
As we will see in upcoming weeks, this change of shape is critical to enzyme function. You have already encountered this once before, with the Sodium/Potassium ATPase.
Daily Challenge
This week you must complete all four forums to be eligible for the weekly summary and summative quiz.
Completion requires that you start a discussion, that your discussion is a minimum of 150 words on topics and at a collegiate level of writing, and that you reply to 3 of your fellow students.
Forum Closes: September 24, 11:55pm.
Completion requires that you start a discussion, that your discussion is a minimum of 150 words on topics and at a collegiate level of writing, and that you reply to 3 of your fellow students.
Forum Closes: September 24, 11:55pm.
Today, I want you to discuss the function of ATP. Do not describe it as an energy currency, instead describe how the addition of phosphates cause a change in the electrochemistry of a protein, and how that affects the conformation of the protein. Use Myosin in muscle cells as your example. We have not covered Myosin, but it is a very easy model for how ATP acts.
This is an easy to follow walk through of the interaction of ATP and Myosin. The site also contains further references.
Myosin ATPase activity: the 'powerstroke' cycle
This is an easy to follow walk through of the interaction of ATP and Myosin. The site also contains further references.
Myosin ATPase activity: the 'powerstroke' cycle
Reference
1. Berg JM, Tymoczko JL, Stryer L. (2002). Biochemistry. W H Freeman, New York. http://www.ncbi.nlm.nih.gov/books/NBK22399/, accessed on September 25, 2012
Subscribe to:
Posts (Atom)