
Daily Newsletter
September 1, 2014 - Polymerization and Amino Acids
This week is an introduction to biochemistry. It is critical that you recall how chemical bonds are formed, and more specifically the idea of covalent bonds (both polar and non-polar) and hydrogen bonds. Biochemistry is based on the fundamentals learned in first year chemistry, but to fully understand it, you will need organic chemistry. So your goal here is not to "master" biochemistry, but instead gain an appreciation and starting understanding of the chemistry of various biochemicals. The first step is to understand the concept of biopolymers and the process of polymerization.
Biopolymers
Proteins, Carbohydrates, Deoxyribonucleic Acid and Ribonucleic Acids are all biopolymers. The suffix bio- deals with life, so it is the root word polymer that we need to discuss. A polymer is a chain, or network of chains, composed of repeating chemical units. These chemical units, or monomers, are generally of the same chemical family, and have the ability to bind together to form larger structures (usually chains). For example, Glucose, a carbohydrate, can bind to Fructose (another carbohydrate) to form the disaccharide sucrose. In the mammalian liver, glucose can be bound into a repetitive chain of glucose monomers to create the polysaccharide Glycogen (poly=many, saccharide=sugar, so many sugars, or a polymer of sugars).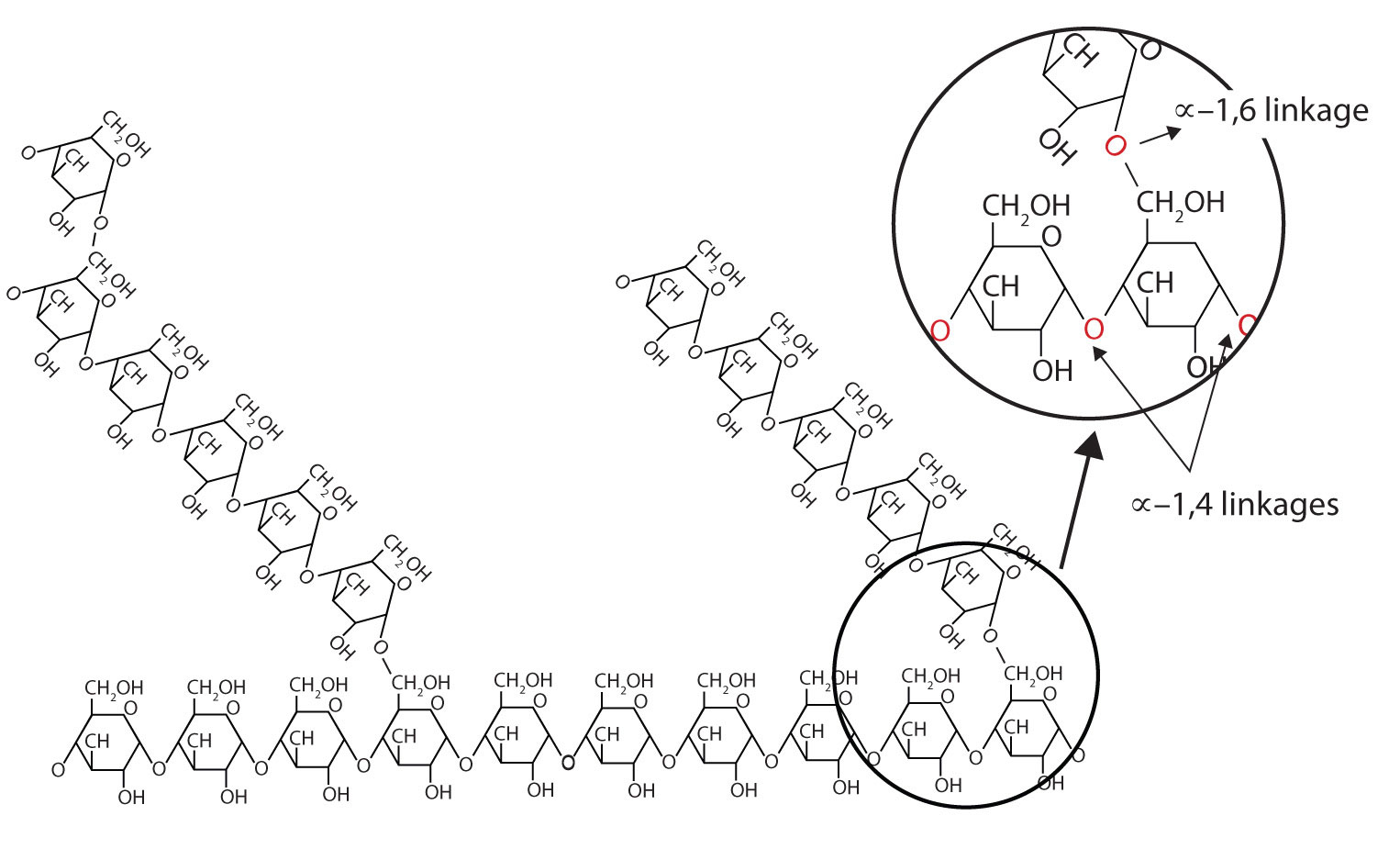
The monomers of biopolymers are linked through condensation reactions (dehydration synthesis) mediated by enzymes. For example, the biopolymerization of glucose into glycogen is handled by the enzyme Glycogen Synthase (note: there are some preliminary steps to get the monomer ready, but the polymerization reaction is handled by Glycogen Synthase).
(Basic Level Description) In dehydration synthesis (condensation), the removal of a hydroxyl from one monomer and a hydrogen from the second allows for the formation of a covalent bond.
 |
| OpenStax College. Dehydration Synthesis [Connexions Web site]. April 4, 2013. Available at: http://cnx.org/content/m44397/latest/Figure_03_01_01.jpg |
Amino Acids and Proteins
Proteins will be a reoccurring topic throughout the semester. They are one of the informational biopolymers. This means that they are composed of monomers (amino acids) that are linked together in specific sequences that are critical to their overall structure and function. [NOTE: It is important to understand the terms monomer and polymer, so make sure you have a good definition of these terms in your notebooks.] To understand proteins, we must first understand their monomeric unit, the Amino Acid.
Remember that all monomers will be chemically similar. In the case of the amino acid, the base molecule of Amino-Chiral Carbon-Carboxyl is the same. The difference in the amino acids comes with the side chain. These functional groups give each amino acid its unique identity and function. The twenty amino acids that are used in natural proteins can be found in the following link: Amino Acid Diagram. Notice that there are four general classes of amino acids with different chemical properties based upon the functional group. NOTE: In the diagram to the right, R represents the radical group. This is the funcational group or side chain. The distinctive characteristics of an amino acid are determined by the R group.
 The base molecule is needed to link amino acids together into a polymer. A condensation (dehydration synthesis) reaction is used to form peptide bonds, the specific bond type that links amino acids together. [NOTE: biopolymers (save for lipids which are not polymers) have specific names for the bonds between monomers.] In the formation of a peptide linkage (bond), you will have a carboxyl and amino group linking together, with water being a product.
The base molecule is needed to link amino acids together into a polymer. A condensation (dehydration synthesis) reaction is used to form peptide bonds, the specific bond type that links amino acids together. [NOTE: biopolymers (save for lipids which are not polymers) have specific names for the bonds between monomers.] In the formation of a peptide linkage (bond), you will have a carboxyl and amino group linking together, with water being a product.Daily Challenge
In the above diagram of the dipeptide (two amino acids connected by a peptide bond), notice how the the R (functional group) is on the same "side" of the molecule.
When you get to organic chemistry, you will discover that there can be rotation around a carbon atom, especial the chiral carbon at the center of each amino acid. What this means is that the R groups can rotate away from each other toward each other. Consider the different types of amino acids, and most notably their possible interactions.
Your challenge today is to discuss how the different types of amino acids (their functional groups) can react with each other. Stay focused on the "types" and not individual amino acids at this time. To focus your discussion, what would happen if you had a chain of all one type? What would happen if you alternated types? How would you form a section of the protein to interact with a hydrophobic environment and a hydrophilic environment at the same time?
No comments:
Post a Comment