Daily Newsletter January 17, 2012
Today's Topic: Proteins
Proteins will be a reoccurring topic throughout the semester. They are one of the informational biopolymers. This means that they are composed of monomers (amino acids) that are linked together in specific sequences that are critical to their overall structure and function. [NOTE: It is important to understand the terms monomer and polymer, so make sure you have a good definition of these terms in your notebooks.] To understand proteins, we must first understand their monomeric unit, the Amino Acid.
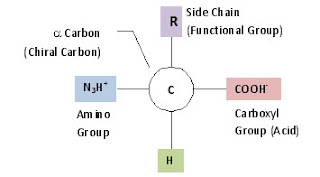
Remember that all monomers will be chemically similar. In the case of the amino acid, the base molecule of Amino-Chiral Carbon-Carboxyl is the same. The difference in the amino acids comes with the side chain. These functional groups give each amino acid its unique identity and function. The twenty amino acids that are used in natural proteins can be found in the following link: Amino Acid Diagram. Notice that there are four general classes of amino acids with different chemical properties based upon the functional group.
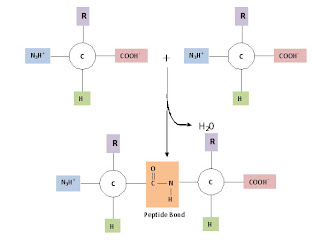
The base molecule is needed to link amino acids together into a polymer. A condensation (dehydration synthesis) reaction is used to form peptide bonds, the specific bond type that links amino acids together. [NOTE: biopolymers (save for lipids which are not polymers) have specific names for the bonds between monomers.] In the formation of a peptide linkage (bond), you will have a carboxyl and amino group linking together, with water being a product.
This linking of amino acids through peptide bonds will create the primary structure of a protein. All of the remaining levels of protein structure will result from interactions between functional groups on the amino acids. Local interactions induces folding into the secondary structures, which will result in other amino acids coming into close contact. This results in a tertiary structure. Finally, you will get a quatrenary structure when multiple individual folded peptide chains come together. Remember that Van der Waals forces (including hydrogen bonds), covalent bonds (disulfide bridges), and hydrophobic interactions will all induce folding. Because of this, environmental factors (such as heat or pH) can influence the folding pattern and shape of a protein.
Daily Challenge: Protein Folding
Today's challenge is tough, and will require you to do some extra reading and research. Hemoglobin is critical to oxygen tranport in many animals. It is an amazing protein that is influenced by body pH and temperature. Minor alterations in hemoglobin can cause radical changes in its ability to carry oxygen. Students are to look at two major changes in hemoglobin:
- When you exercise, your muscles warm up and give off acidic metabolic waste. How does higher temperatures and acidic conditions change the shape of hemoglobin, and theirfore its ability to hold oxygen? Hint: Look up an oxygen dissociation curve and its explaination.
- Sickle Cell Anemia is the result of a small mutation in the gene that codes for β-Hemoglobin. Only one amino acid is different. Explain how and why a single amino acid change can cause the problems seen in sickle cell anemia. Hint: Look up the pathophysiology of sickle cell anemia as a starting point.

No comments:
Post a Comment