 Daily Newsletter
Daily Newsletter
October 8, 2012 Harvesting Photons
This week brings us to Photosynthesis. The focus will be on eukaryotic photosynthesis, which takes place in the chloroplast. The activity of photosynthesis is broken down into two major sets of reactions: Light Dependent and Light Independent.
During the Light Dependent reactions, photons will be used to excite electrons in various pigments. These excited electrons will be used to produce reduced electron carriers (NADPH + H+) and ATP. During the light independent reactions, CO2 will be reduced and incorportated into organic compounds (carbon fixation). This will lead to the formation of glucose though the Calvin Cycle (metabolic pathway).
Today, your goal is to understand how photons are used to excite pigment electrons.
In photosynthesis, the cell will generate reducing power by using a photon to excite an electron. You will recall from chemistry that that electron excitation occurs when an electron jumps to a higher energy state (a higher shell).
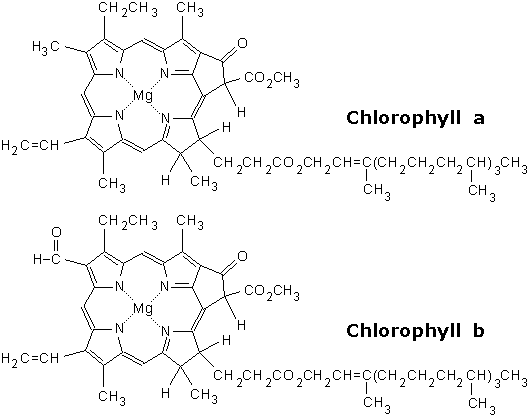
Photoexciation is one of the principle ways to excite an electron, and we can do this in a modern lab fairly easily. In nature though, you require a structure that can "focus" the photon's energy. Chlorophyll provides such a structure. As a pigment, the structure of chlorophyll is built to absorb specific wavelengths of light, and then excite a set of electrons.
Note that at the center of the chlorin ring (the organic ring structure) is a Magnesium ion, which can be considered the focal point of the photoexciation. Remember our discussions about the respiratory chain, specifically NADH dehydrogenase? Fe-S complexes were used for redox reactions with the protein. Magnesium, like iron, can easily go through redox reactions. As a point of reference, visualize the photon exciting the magnesium ion.
Chlorphylls are arranged in systems, and once photoexcitation has occurred, the energy is transferred by resonant energy transfer to a Reaction Center.
Resonant energy transfer? What's that?
This is a concept from quantum physics. It describes how energy is transferred between chromophores (e.g., Chlorophyll). One chromophore is struck by a photon and is excited. Instead of giving off light (i.e., fluorescence), the chromophore transfers the energy to a neighboring chromophore (if you car curious, this occurs through dipole-dipole coupling). As with any energy transfer, the 2nd law of thermodynamics applies. The amount of energy lost is inversely proportional to the distance between chromophores (it is very energy efficient between close neighbors). The important thing to remember here: we don't move electrons, we just transfer energy. This is possible due to the structure of the chromophores (i.e., pigments).
Energy is passed between chromophores in an antennae complex toward a reaction center. The reaction center is formed by a pair of chlorophyll molecules, and is chemically active. The reaction center can undergo redox reactions. There are two specific reaction centers known in plants, the P680 and P700. These names refer to the maximum red absorbance wavelength for these two molecules. The P680 reaction center is found Photosystem II, while P 700 is found in Photosystem I. (Photosystem is the name given to a reaction center and the associated electron transport chain. The naming is historic: Photosystem I was found before Photosystem II).
The reaction centers gather the energy absorbed by the surrounding chlorophyll. These reaction centers then undergo a charge separation, which is a form of redox reaction that donates high energy electrons to a waiting quinone. The image below is of photosystem II.

1) Notice in the center there is a group f structures with the code Pheo. This represents a Phenophytin, which is a chlorophyll without the magnesium in the center.
The paired chlorophylls become a paired phenophytin. This change (loss of the magnesium) is a where the absorbed energy is converted to a redox potential. The energy is handed off to a quinone (Q).
You will recall ubiquinone, which we saw in the eukaryotic respiratory chain. Ubiquinone was a type of quinone. So this is a hydropobic mobile electron carrier (i.e., it can travel in the lipid portion of a phospholipid bilayer). The quinones take the harvested reducing potential to electron carriers for processing.
2) Notice that the reduction of the quinone requires Fe. Recall that Fe-S complexes were used in the respiratory chain for redox reactions. Iron is again used to help faciliate redox reactions.
3) Photosystem II has a way to recharge the system with electrons and hydrogen. The reaction center of photosystem II has hydrolytic ability; it can break water. The hydrogens, and their electrons, join photosystem II, recharging the reaction center (they convert pheophytin back to chlorophyll). In the process, they release oxygen (O2).
This is critical: the individual pigments do not undergo redox reaction; instead they transfer energy to neighboring chromophores (i.e., pigments). The reaction center undergoes redox reactions, passing electrons to ubiquinone. The reaction center lost electrons, and must replace these electrons. Photosystem II handles this replacement by using H2O. (Connection: H2O was created due to the reduction of the final electron acceptor O2. Now H2O is used to replace the missing electrons in the P680 reaction center, and O2 is given off).
The harvested electrons will be used to either make ATP (chemiosmosis) or to reduce NADP+ to NADPH + H+. NOTE: Photosynthesis uses Nicotinamide Adenine Dnucolotide Phosphate. NAD+ is used in catabolic reactions, while NADP+ is found in anabolic reactions.
Photosystem II & I - Non-cyclic

Daily Challenge
Explain in your own words how photon energy is harvested and converted into reducing power. Include in your discussion a description of the above electron transport system involved in producing NADPH + H+ and ATP.Link to Forum


No comments:
Post a Comment