Daily Newsletter January 31, 2012
Today's Topic: The Nucleus
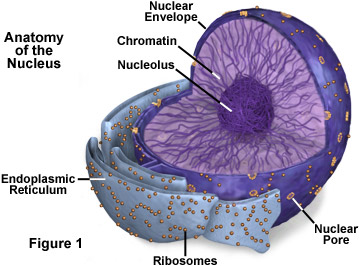
It can be said that the nucleus defines the eukaryotic cell (question: why?). The nucleus is a central structure present in eukaryotic cells, and is the site where we find the cell's DNA. It is a highly regulated structure, and one function is to ensure the protection and stability of the cell's genetic information.
This is a double membrane bound structure, meaning there are two lipid bilayers that make up the nuclear envelope.The outer layer of the nuclear envelope gives rise to the endomembranous system, which includes the Endoplasmic Reticulum and the Golgi Apparatus.
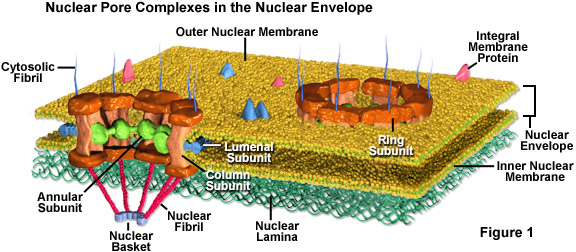
To gain access to the inside of the nucleus, you must first move through nuclear pores (which are highly regulated). Click on the link for more information on the nuclear pore complex.
Here is a video of the nuclear pore complex in action. It does a good job with the visual, but there is one thing you need to realize with all gated portals in the cell: to get through, you must have the correct molecular key!
The endomembranous system, which includes the Endoplasmic Reticulum and Golgi Apparatus is a continuation of the outer membrane of the Nuclear Envelope. While the nucleus deals with DNA, and the transcription of DNA into RNA, the endoplasmic reticulum will deal with translation of RNA into Proteins and the production of lipids. The Golgi Apparatus will deal with the process, storage and packaging of proteins and other products. Why is it important that we have different compartments for the processing, storage and packaging of proteins?
Here is a good visual of the Golgi Apparatus.
Daily Challenge: Today, you can choose to write about of two topics (if you feel ambitious, you can write about both). 1) Describe the nucleus, and in your own words, the action of the nuclear pores; 2) Describe the endomembranous system, and the production and processing of proteins.