Study Skills
It is always helpful to reflect on your study skills and see if there are ways you can improve your learning. Today, consider whether you maintain focus when you sit down to study. |
| Figure 1: chemical structure of an amino acid |
Amino Acids and Polymerization
Amino Acids and Proteins Proteins will be a reoccurring topic throughout the semester, so for today let's first review amino acids and the basics of biochemical polymerization. They are one of the informational biopolymers. This means that they are composed of monomers (amino acids) that are linked together in specific sequences that are critical to their overall structure and function. [NOTE: It is important to understand the terms monomer and polymer, so make sure you have a good definition of these terms in your notebooks.] To understand proteins, we must first understand their monomeric unit, the Amino Acid.Remember that all monomers will be chemically similar. In the case of the amino acid, the base molecule of Amino-Chiral Carbon-Carboxyl is the same. The difference in the amino acids comes with the side chain. These functional groups give each amino acid its unique identity and function. The twenty amino acids that are used in natural proteins can be found at the following link: Amino Acid Diagram. Notice that there are four general classes of amino acids with different chemical properties based on the functional group. NOTE: In the diagram to the right, R represents the radical group. This is the functional group or side chain. The distinctive characteristics of an amino acid are determined by the R group.
 |
| Figure 2: Polymerization of amino acids by a condensation reaction. |
The base molecule is needed to link amino acids together into a polymer. A condensation (dehydration synthesis) reaction is used to form peptide bonds, the specific bond type that links amino acids together. [NOTE: biopolymers (save for lipids which are not polymers) have specific names for the bonds between monomers.] In the formation of a peptide linkage (bond), you will have a carboxyl and amino group linking together, with water being a product.
Protein Self-Assemble (Folding)
Let's now further examine the importance of functional groups by discussing how they help form the working shape of a protein by causing a chain of amino acids to fold.The primary structure of a protein is a chain of amino acids. Due to how proteins form, one end of the chain will end in an amino group (N-terminus), which the other end will have a carboxyl group (C-terminus). In Between these two terminal points will be a wide variety of amino acids.
The side chains of neighboring amino acids will begin to interact. They could be pulled toward each other, be repelled, or have nothing happen. Remember that the functional groups can twist around the chiral (central) carbon of the amino acid, so repulsion may just force the side chains to opposite sides of the chain (remember, you are dealing with 3-D structures here). The amino groups and carboxyl groups, even though they are part of the backbone, also retain polarity. Thus they can also be involved in the folding.
These interactions start the formation of the secondary level of protein structure. The two most common types of secondary structures are the alpha helix and the beta pleated sheet. These two types of secondary structures will help explain how the amino acid side chains start the folding process.
 |
| Figure 4: α-helix |
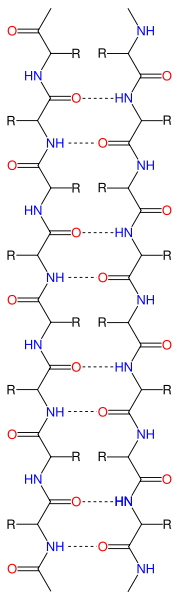 |
| Figure 4b: β-pleated sheet |
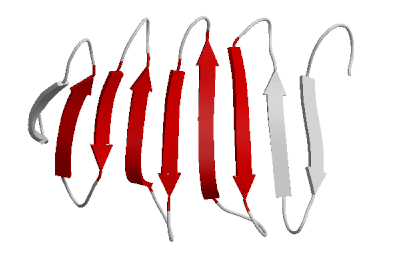 |
| Figure 4a: β-pleated sheet |
 |
| Figure 5: Tertiary Structure |
a protein in primary and then tertiary structure.
Notice that the secondary structures are visible, but even these have been folded into each other.
 |
| Figure 6: Disulfide bridge |
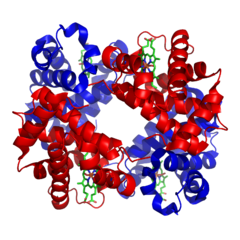 |
| Figure 7: Quaternary structure (Hemoglobin) |
NOTE: Some proteins are functional in the tertiary structure, but others are only functional when you have multiple individual proteins forming the quaternary structure.
Denaturation: The protein is held together primarily through electrostatic interactions. What happens when a protein warms up? It starts to unfold. Why? The electrostatic interactions weaken as the kinetic movement of the atoms increases. Acids and bases, with their charged H+ and OH- also disrupt these electrostatic interactions. Secondary and tertiary structures begin to change conformation. Most notably, they unfold. To denature a protein is to unfold it....but....
What if you only apply a mild heat, let's say your muscles warm up due to exercise. What happens to the proteins? What happens to hemoglobin when it passes through a warm temperature? Your muscles are also metabolizing, and as we will see, produce acids. What does this do? So, is denaturation all or nothing?
The tertiary and quaternary structures all have a specific electrochemical profile.
What happens if you add a charged particle/compound to a protein? What happens if I add a new positive charge? Answer: The protein will change shape (conformation).
What will this due to the function of the protein? It could actually activate the protein, but it could also deactivate the protein. This will be a discussion a little later in the semester, but I want you to start thinking about the implications.
Example: Hemoglobin
Adult hemoglobin is a quaternary protein, composed of two α-subunits (tertiary proteins) and two β-subunits (tertiary proteins). Each subunit contains a heme group which bears an Fe+2. The heme is a prosthetic group of the individual proteins; without the heme, the subunit is non-functional. We will see further examples of prosthetic groups and other factors as we go through the semester. NOTE: We are looking at adult hemoglobin.
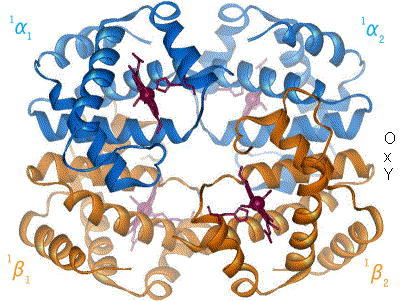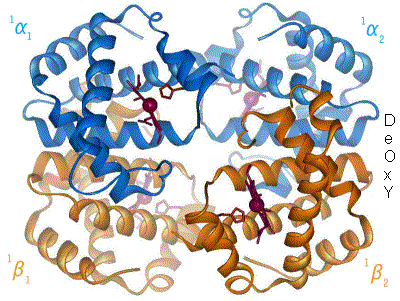 |
| Figure 8: animation of hemoglobin t-r state transformation, made by en:User:BerserkerBen http://en.wikipedia.org/wiki/File:Hemoglobin_t-r_state_ani.gif |
The folding of the protein is critical to its function. Even a small error in the primary structure can cause significant changes to the overall structure, and therefore changes to the function.
| Figure 9: Normal vs. Sickle β-Hemoglobin http://helicase.pbworks.com/f/1225738609/hemoglobins.GIF |
The quaternary protein has now changed shape by opening up. Sickle hemoglobin has the ability to bond to other sickle hemoglobins. This is due to hydrophobic interactions among valine, and the loss of a salt bridge (ionic interaction). The image below shows the result of this interaction between sickle hemoglobin: clumping.
 |
| Figure 10: Interaction between hemoglobin molecules http://evolution.berkeley.edu/evosite/evo101/images/hemoglobin.gif |
Below is an image comparing the shape of the normal erythrocyte (red blood cells) and the sickle variation.
 |
| Figure 11: Normal vs. Sickle Erythrocytes (Red Blood Cells) |
Challenge:
Here are two challenges to help you think about protein structure.Challenge 1
Hemoglobin is a protein that is constantly changing shape due to the presence or absence of oxygen. It also changes shape when in acidic environments or hot environments (think of your muscles after a work out). The effects of temperature and pH are described in the Oxygen Dissociation Curve. Temperature and pH disrupt electrostatic interactions, allowing for proteins to denature. Using the concept of denaturation, explain why more oxygen is released when the pH lowers and temperature increases. How would this be of benefit to the body?Challenge 2
Antibodies help to defend the body against infections. They work by binding foreign chemical markers called antigens. The diversity of antigens is staggering, and cells that make them (B lymphocytes) have the ability to undergo somatic recombination (programed changes to DNA) to increase the variability of antibodies. Of specific importance is the variable domain: |
| Figure 12: Structure of an antibody http://www.accessexcellence.org/RC/VL/GG/ecb/ecb_images/04_32_antibody.jpg |
In the above diagram, look at the Variable Domain of the Light Chain (VL). Notice 3 areas drawn in red, and referred to as the Loops that Bind Antigens. These are the areas where Somatic Recombination can change the nucleotides present in the gene. If I change the nucleotides (as with hemoglobin above), I can change the amino acids in the primary sequence. Explain how changing the primary sequence in these loop regions can change the binding affinity of an antibody to an antigen, and why this is beneficial to the body.
No comments:
Post a Comment