Daily Newsletter
September 25, 2013 Enzymes
Enzymes are the workhorses of the cell. They catalyze reactions, meaning they decrease reaction activation energy. It is critical to learn how enzymes function. To start with, here are some things you need to remember.
- First thing to remember, Enzymes are Proteins!
- So they are constructed on ribosomes.
- Their structure is determined by electrostatic interactions.
- Their shape can change when things bind to it.
- They can be denatured.
- Second, they act as catalysts.
- They lower activation energy by bringing molecules together in the correct alignment, and can induce molecular tension to cause the reaction.
- Though their shape may change during the chemical reaction, the enzyme is left essential unchanged at the end of the reaction.
Every enzyme has an active site. This is the place where the substrate(s) will bind to the enzyme. The active site must have a shape that loosely fits the substrate, and the electrochemical pattern of the active site must compliment the electrochemical pattern of the substrate. When they bind, you get an Enzyme Substrate complex. Below is a basic cartoon about the process:
This enzyme-substrate complex is critical, and it is likely that you will see the development of an intermediate during the process. As the substrate "binds" to the active site, the overall enzyme will change shape. This conformational change is part of how the enzyme lowers the activation energy of the reaction. In the case of a synthesis, the enzyme will force the two compounds into close association, while in a break-down reaction, the enzyme may appear to bend the molecule at a breaking point. Here is a quick video that shows the brief conformational change that helps to induce the reaction: http://youtu.be/V4OPO6JQLOE
Conformational changes alone are not the only part in inducing a reaction. You will also find that proteins can have prosthetic groups that aid them in their action. For example, you can a Heme group with Iron that can hold Oxygen in red blood cells. Some digestive enzymes use Chromium to help in their action. Many metabolic pathways will contain Electron Carriers needed for redox reactions.
Enzyme Regulation
Cells are filled with enzymes, and they are constantly working to maintain cellular homeostasis. Some of the enzymes though are produced in an inactive form. We need them, but not always. We are also able, though chemical signals, to turn off some enzymes. Last week, we looked at signal transduction, converting a chemical signal into a change in cellular physiology. We saw the action of protein kinases and other primary and secondary signals. One purpose of the protein kinases was to alter the activity of proteins in the cell.
Beyond an active site, many enzymes have an allosteric site (regulatory site). This is located away from the active site. When the regulatory compound "binds" to the allosteric site, the enzyme changes shape. Most critically, the enzyme alters the shape of the active site. An allosteric activator is a signal that opens the active site, while an inhibitor closes the active site. The image to the right shows an enzyme in the active and inactive state. Notice that the active site changes when the allosteric activator is bound to the enzyme. In the case of this enzyme, there are two different ways to regulate: Phosphorylation, which would have occured due to a chemical signal such as cAMP, and the binding of ATP at an allosteric site.
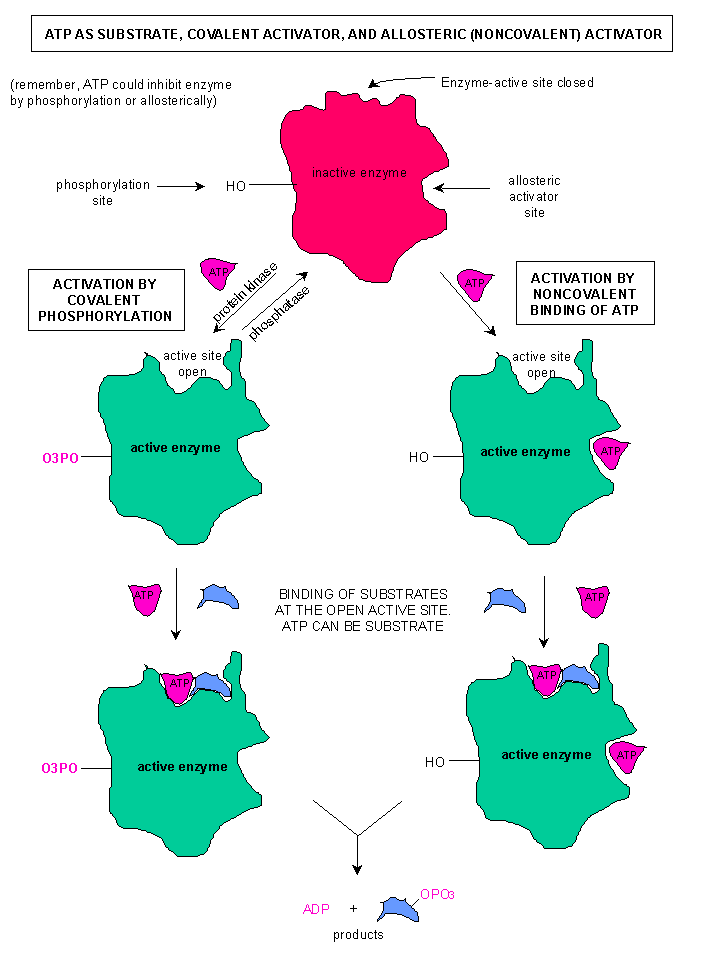 It is not uncommon to have multiple ways to regulate an enzyme, and some enzymes that can be activated and deactivated as needed. A good example of this is with the enzyme Phosphofructokinase, a critical enzyme in glycolysis. This enzyme catalyzes an energy consumptive reaction that is a "no turning back" point in glycolysis.
It is not uncommon to have multiple ways to regulate an enzyme, and some enzymes that can be activated and deactivated as needed. A good example of this is with the enzyme Phosphofructokinase, a critical enzyme in glycolysis. This enzyme catalyzes an energy consumptive reaction that is a "no turning back" point in glycolysis.Before we talk about phosphofructokinase, we need to talk a little of why we regulate proteins. Two memes I want you to remember: Cells are masters at energy conservation & Cell do not carry out unneccessary reactions. Both concepts are related, and they will help you understand why proteins are regulated. While there are energy consumptive pathways, the main reason we regulate proteins and pathways is due to products. Remember our discussion of equilibrium. We need to use products (products must become the next substrate). If we build up product, the reverse reaction becomes more likely. To ensure that we do not shift equilibrium, we try to avoid product build up. So, we want to stop reactions when we have an adequate to abundant supply of a compound.
The goal of our energy harvesting pathways, of which glycolysis is part, is the production of ATP. So ATP becomes a regulator. Phosphofructokinase has an allosteric site that is specific for ATP. When there is an abundant supply of ATP, some of the ATP binds to phosphofructokinase and changes its shape. Specifically, it closes the active site.
 The presence of AMP in the cell is a singal that we need to unlock our energy harvesting pathways, specifically, unlock phosphofructokinase. In this case, AMP acts as an activator to reverse the inhibitory effect of ATP. To the left is a diagram of phsophofructokinase. The area labeld "Regulatory Site" is the Allosteric site.
The presence of AMP in the cell is a singal that we need to unlock our energy harvesting pathways, specifically, unlock phosphofructokinase. In this case, AMP acts as an activator to reverse the inhibitory effect of ATP. To the left is a diagram of phsophofructokinase. The area labeld "Regulatory Site" is the Allosteric site. In lecture, we will discuss inhibition at the active site.
Additional Resources
Enzymes at Blobs.org - This site has a good review of enzymes, including regulation, inhibition, kinetics and enzyme function at different temperatures. The images presented on the site are extremely helpful.Daily Challenge
Discuss the concept of enzymes using angiotensin converting enzyme (ACE) as your example. ACE is a medically important enzyme that is involved in an imporant signal system in the body that helps to regulate water balance and the kidneys. There is a group of drugs known as ACE inhibitors that affect the activity of this enzyme. Your goal is to discuss the enzyme, including its regulation and inhibition.Note: ACE converts the plasma protein Angiotensin I into Angiotensin II (consider this the active form). Angiotensin II has numerous effects, including causing the kidneys to retain sodium and water, increasing blood volume, and thus increasing blood pressure. ACE Inhibitors are used with people who have high blood pressure.

No comments:
Post a Comment