Daily Newsletter
September 17, 2013 The Basis of the Cell Membrane.
Nature of the Cell
The cell theory describes the importance of the cells to biologists. How important is it? Well the first part of the cell theory answers that: All known living things are made up of one or more cells. But what ultimately is a cell? What takes place in one? Why are they so important?It will take a few discussions to get to all these questions, but there is a starting point. As a basic description, the cell is a self-managing, self-contained chemical factory. Cells take in materials, use these for energy and building blocks, and then produce materials to keep the cell healthy, harvest nutrients, eliminates waste, and produces products. The cell has many components to accomplish these tasks: DNA for management, chemical signals to send messages, enzymes for chemical activity, etc....
Let's look at the analogy of a chemical factory: Inside a factory, there are going to be different processing places for different chemicals. There are going to be pathways of pipes going between vats and other structures. Taking a further look back, there is a building, with trucks coming and going.
Inside of the cell, chemical reactions will be taking place. Outside the cell, chemical reactions are taking place. Are they the same chemical reactions? One of the foundations of cells is that the inside of a cell is a spatial area with a defined concentration of a variety of chemicals that is distinct and different from the chemical concentrations found outside of the cell.
So, there is an inside and outside of a cell, and they are different.
You will hear me say again and again that the cell membrane is the defining structure of a cell. Why? Because it establishes the boundary. When you have a cell membrane, you can have an inside as opposed to an outside of a cell. If you loose that membrane, you start moving to a full equilibrium between the inside and outside of the cell. If you loose the membrane, or it gets holes, the cell dies. The cell dies when you cease to have an inside vs. an outside.
The fastest way to kill a cell is to poke holes in it.
Lipids
Lipids are an odd group of biomolecules. Proteins, Carbohydrates and Nucleic Acids are all formed through polymerization reactions; they have monomeric units that join to make polymers. Lipids do not polymerize, and they have no monomers. Instead, Lipid is a word that defines a class of hydrophobic organic compounds found in living systems. There are a number of important groups of Lipids, such as the triglycerides, phospholipids and cholesterols. Today, we are going to concentrate on the triglycerides and the phospholipids.Both triglycerides and phospholipids possess a glycerol molecule and fatty acids.
 Glycerol is a 3 carbon compound that we will see from time to time. It acts as the backbone or schaffold of the triglycerides and phospholipids. As you can see, on each carbon atom, there is a hydroxyl group (-OH). This hydroxyl group is where other molecules can bond. Another thing to note is that there is free rotation around the carbon atoms. When you take organic chemistry, you will learn more about free rotation, why it is important, and how it can affect a molecule. For now, just note that there can be free rotation.
Glycerol is a 3 carbon compound that we will see from time to time. It acts as the backbone or schaffold of the triglycerides and phospholipids. As you can see, on each carbon atom, there is a hydroxyl group (-OH). This hydroxyl group is where other molecules can bond. Another thing to note is that there is free rotation around the carbon atoms. When you take organic chemistry, you will learn more about free rotation, why it is important, and how it can affect a molecule. For now, just note that there can be free rotation.Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. To the left is a diagram of palmitic acid, a typical saturated fatty acid.
 chemicals are depicted. Below the first depiction is a molecular model. Carbon atoms are in black, and the white balls are hydrogens. What you will begin to recognize in these diagrams is that there are only single bonds between the carbon atoms. This means that the maximum number of hydrogen atoms are attached to the fatty acid. In other words, they are saturated with hydrogen.
chemicals are depicted. Below the first depiction is a molecular model. Carbon atoms are in black, and the white balls are hydrogens. What you will begin to recognize in these diagrams is that there are only single bonds between the carbon atoms. This means that the maximum number of hydrogen atoms are attached to the fatty acid. In other words, they are saturated with hydrogen.In contrast, an unsaturated fatty acid does not have the maximum number of hydrogen atoms. This occurs when double bonds (two electrons from each carbon are shared) occur in the carbon chain. To the right is a diagram of oleic acid.
 Note that there is a double bond in the carbon chain. Notice that the chain is bent, or kinked. This creates a very different structure for lipids that carry unsaturated fatty acids.
Note that there is a double bond in the carbon chain. Notice that the chain is bent, or kinked. This creates a very different structure for lipids that carry unsaturated fatty acids.As a general rule, saturated fatty acids are solid at room temperature, and unsaturated fatty acids are liquid in room temperature. But one thing is the same in both: the carbon chains are HYDROPHOBIC!
In a triglyceride, the carboxyl end of the fatty acid will react with the hydroxyl end of the glycerol.
 As you can see in the diagram, the two molecules are joined together through an oxygen molecule. As with other biosynthetic reactions, this is a dehydration synthesis (water is released). The resulting bond, as noted in the diagram, is an ester bond. You will learn more about this bond in organic chemistry, so for now I just want you to remember the general look of it. Why is this so critical? Because not all living organisms make triglycerides and phospholipids this way. Member of domain Archaea use an ether bond.
As you can see in the diagram, the two molecules are joined together through an oxygen molecule. As with other biosynthetic reactions, this is a dehydration synthesis (water is released). The resulting bond, as noted in the diagram, is an ester bond. You will learn more about this bond in organic chemistry, so for now I just want you to remember the general look of it. Why is this so critical? Because not all living organisms make triglycerides and phospholipids this way. Member of domain Archaea use an ether bond.So, what is the difference between a triglyceride and a phospholipid?
 Recall the look of the glycerol, and note that there are three locations where an ester bond can be formed. In a triglyceride, a fatty acid will be bound to each of the carbon atoms by ester bonds. Tri- means three, so we have three fatty acids attached to the glycerol. The image to the right is an example of a triglyceride, and please note, you can have more than one type of fatty acid in a triglyceride.
Recall the look of the glycerol, and note that there are three locations where an ester bond can be formed. In a triglyceride, a fatty acid will be bound to each of the carbon atoms by ester bonds. Tri- means three, so we have three fatty acids attached to the glycerol. The image to the right is an example of a triglyceride, and please note, you can have more than one type of fatty acid in a triglyceride.The phospholipid in contrast only has two fatty acids. The third binding location will be used for a "phosphate head". This head contains a phosphate group and usually a diglyceride and some small charged organic structure.
 Phosphytidyl choline is a commonly studied phospholipid that uses choline as the charged organic structure. NOTE: both the phosphate group and the organic structure carry a charge. This phosphate head is charged, thus it is hydrophillic (water loving). The phospholipid contains non-polar, hydrophobic fatty acids (usually referred to as the tails) and a polar, charged, hydrophillic head. This molecule is both hydrophobic and hydrophillic. Amphiphathic is the word we use to describe a molecule with both hydrophobic and hydrophillic properties. A main use for the phospholipid is in biological membranes, as shown in the image to the right.
Phosphytidyl choline is a commonly studied phospholipid that uses choline as the charged organic structure. NOTE: both the phosphate group and the organic structure carry a charge. This phosphate head is charged, thus it is hydrophillic (water loving). The phospholipid contains non-polar, hydrophobic fatty acids (usually referred to as the tails) and a polar, charged, hydrophillic head. This molecule is both hydrophobic and hydrophillic. Amphiphathic is the word we use to describe a molecule with both hydrophobic and hydrophillic properties. A main use for the phospholipid is in biological membranes, as shown in the image to the right.Fundamental Structure of the Cellular Membrane
The basic structure of the cellular membrane is composed of Phospholipids. The amphipathic nature of phospholipids means that they will naturally associate with one another. Specifically, the hydrophobic tails want to be with other hydrophobic compounds, and exclude polar compounds (Hydrophobic Exclusion-This is a good concise discussion of the topic by Stephen T. Abedon, Ph.D. at Ohio State). Below is a brief movie that shows what happens when phospholipids in water begin to interact.Phospholipid Movie: Bilayer formation through molecular self-assembly
 The resulting phospholipid bilayer is polar (hydrophilic) on the outside, while the middle is non-polar (hydrophobic). The sides interact with water, but the middle excludes polar substances. This creates a selectively permeable barrier, and is the basis of the membranes function. Changing phospholipids and adding sterols (like cholesterol) will change the integrity and stability of this basic membrane structure.
The resulting phospholipid bilayer is polar (hydrophilic) on the outside, while the middle is non-polar (hydrophobic). The sides interact with water, but the middle excludes polar substances. This creates a selectively permeable barrier, and is the basis of the membranes function. Changing phospholipids and adding sterols (like cholesterol) will change the integrity and stability of this basic membrane structure.Selective permeability means that only certain classes of chemical can make it through the phsopholipid bilayer. For other chemicals, we need to provide a protein to serve as a pore, channel or transporter.
In general, there are two ways that a chemical can be moved across the membrane: Down the chemical's concentration gradient (diffusion), or Against the chemical's concentration gradient. When a chemical moves down it's concentration gradient, we do not need to add energy to the process. The concentration gradient and the kinetic energy of the molecule (Brownian Motion) provided the needed energy. We call this form of movement Passive Transport.
There are three basic forms of passive transport through the membrane:
- Diffusion - Using the inherent Brownian motion of molecules, chemicals move from points of high concentration to points of low concentration.
- Every chemical has a unique concentration gradient.
- The concentration gradient of one molecule will not interfer with the concentration gradient of another molecule.
- What interfers is the ability to move across the cellular membrane (phospholipid bilayer).
- Molecules with high polarity, ions, and large molecules are excluded by the phospholipid tails, and thus can not cross.
- Water*, CO2, O2, and nonpolar compounds (lipids) can cross though diffusion.
- Water moves through very slowly; water moves across more readily due to porins (protein pores) possessed by cells to make sure water and small polar compounds can cross readily.
- Water goes to where the party is, meaning it will move to a compartment that has a higher solute concentration.
- Since we are talking about two fluid compartments on either side of a membrane, we are ultimately talking about relative solute concentrations.
- REMEMBER: you are looking at two fluid compartments, so when we are looking at osmosis, we are discussing the movement of water across a selectively permeable membrane from one fluid compartment to a second fluid compartment.
- In biology we always use the inside of the cell as our reference, so:
- An isotonic fluid has the same solute concentration as the inside of the cell.
- A hypertonic solution has a solute concentration that is higher than the solute concentration inside the cell.
- A hypotonic solution has a solute concentration that is lower than the solute concentration inside the cell.
- Remember that we are looking at solute concentrations, not the concentration of a single chemical.
- Diffusion gradients are specific for each chemical.
- Osmosis is determined by total solute concentration on each side of a selectively permeable membrane.
- The cell provides a protein channel or pore for the chemical to pass through.
- Each channel or pore is specific to a single chemical or set of chemicals.
- Since this is an protein (enzyme) mediated action, the ability to transport follows standard enzyme kinetics.
- The core idea about facilitated diffusion following enzyme kinetics is that diffusion becomes limited by the number of channels or pore available.

- Note: You will generally build up a large concentration of a compound on one side of the membrane. We will refer to this as a steep gradient (high on one side, low on the other).
- The facilitator proteins will allow molecules from the HIGH side to move toward the LOW side; hence the use of the word diffusion.
- Rarely will the channel or pore (facilitator) allow transport in the reverse direction for the same ion.
Osmosis Movie
Important Memes
Occassionly, there will be a phrase that I want you to remember to help you as you move through biology.- Water goes to where the party is! (This is a great way to remember in what direction water will flow across a selectively permeable membrane in response to changes in osmolarity, aka, solute concentration).
- The phospholipid bilayer establishes the structure of the membranes, but the proteins provide the function. (The proteins embedded in the membrane will determine the functional capabilities of the membrane).
- When something is added to a protein, the protein changes shape.
Daily Challenge: Nature of the Cell
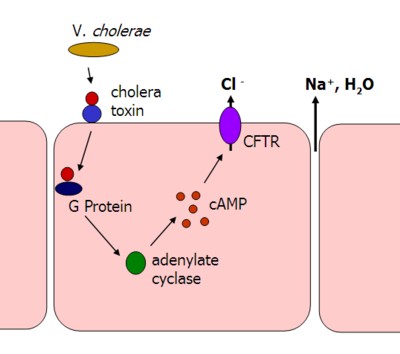
A critical concept for biologists and biochemists is that we never see a reaction or molecular movement that results in a final equilibrium, as you would see in a chemistry lab. The cell is constantly moving substances across the membrane, actively maintaining concentration gradients, and quickly using products of chemical reactions. Water is one of the few compounds we do not use/regulate in this way. Instead, water is regulated by solute concentrations. When solutes are moved from one fluid compartment to another, water follows. The cholera toxin offers a great example of how water moves when we move solutes.The cholera toxin forces intestinal endothelia cells (cells that line the intestine) to purge Cl- into the lumen of the intestine (hollow tube). Water follows. The end result, diarrhea.
Your goal today is to reflect on two different concepts: 1) the formation of fluid compartments (at minimum the inside and outside of a cell) as critical to the life of the cell, and 2) how the cell maintains a dynamic equilibrium among all the chemicals it uses.

No comments:
Post a Comment