 Daily Newsletter
Daily Newsletter
October 1, 2013 Glycolysis part 2
Today we review the second half of the glycolytic pathway. Below is one of the best images of glycolysis online. Feel free to use this as a reference when working through glycolysis.
 |
| Karp, G. (2009). Cell and Molecular Biology: Concepts and Exerpiments, 5th ed. Wiley, New York, p. 108. Image initially found online. The image may be from the 4th edition of the book. |
Step 6 is catalyzed by Glyceraldehyde Phosphate Dehydrogenase. The word dehydrogenase explains the action. We are removing hydrogens. When we remove hydrogens, we also remove electrons. Dehydrogenases are responsible for oxidizing a substrate. So this is a redox reaction. You can also tell this because our electron carrier NAD is being reduced as a by product of this reaction. So we remove electrons (energy) from Glyceraldehyde 3-P and give electrons (energy) to NAD, forming NADH.
This is the single largest change in energy throughout glycolysis. This is a major harvesting of energy (we harvest the most energy using redox reactions). Notice though that the free energy change is +1.5; so why do we say it is an energy harvesting step? REMEMBER: free energy calculations are based on isolated reactions. This is not a favorable reaction in isolation. Molecules do not like to give up electrons. With enzymes and energy carriers though, this is a favorable reaction. Approximately 100 kcals/mol is actually harvested in this step. That energy is in the form of electrons that are given to the carrier NAD, forming NADH+ +H+(NOTE: The 2 electrons, and therefore 2 hydrogen protons, are harvested. Only one hydrogen binds to NAD+, while the other is free in the cytosol).
Notice that we are also adding a phosphate. But why? We do not use ATP to add this phosphate. We use an inorganic phosphate. One reason is to maintain the stability of the molecule. When substrates are oxidized, the molecule becomes unstable. Sometimes the instability is needed for the next reaction, but sometimes the instability is just a little too much. There is an intermediate here that is unstable, so we add Phosphate to stabilize the product.
But notice, the new phosphate is highlighted in yellow. Why? This is a notation used by the artist to represent a high energy phosphate. On either end of the 1,3 bisphophoglycerate there is a phosphate group, a -2 phosphate group. You have negative charges being held in close association. Is this electrically stable? No, the negative charges want to push away from each other. Thus we get a "high energy" bond (one that is stable, but easily broken).
In step 7, the enzyme Phosphoglycerate Kinase is going to take the high energy phosphate of 1,3-Bisphosphoglycerate and add it to ADP. This allows for the generation of ATP by substrate phosphorylation. The term substrate phosphorylation often causes confusion, but it is very simple. A substrate level phosphorylation means that a high energy phosphate on the substrate is used to regenerate ATP from ADP. You can see that this reaction is considered spontaneous, this is again due to the action of a high energy phosphate. The phosphate on carbon 1 wants to get away from the phosphate on carbon 2. This amount of molecular tension, when released, will yeild a great deal of energy in an isolated reaction. In a coupled reaction, this energy is going to be used to form the phosphoanhydride between the β-phosphate (2nd phosphate from the ribose) and the new γ-phosphate (3rd phosphate from the ribose).
Step 8 is catalyzed by Phosphoglyceromutase (aka Phosphoglycerate Mutase). The core name of the mutase indicates the action, namely that we are altering the molecule. This enzyme is part of the isomerase class of enzymes (so the substrate and product have the same chemical formula), but in this case it is the moving of a functional group or side group. In this case, it is the movement of the phosphate from the 3rd
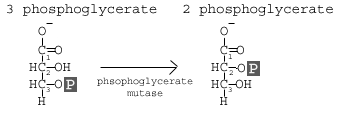 carbon to the 2nd carbon. This is actually a coupled reaction: addition of a 2'-phosphate coupled to the removal of the 3'-phosphate. What does this mean? It means we bring in a new phosphate for the 2nd carbon and remove the phosphate from the 3rdcarbon; the phosphate is not moved, but replaced. If you think about it, this explains the free energy change and why it is not a spontaneous reaction in isolation.
carbon to the 2nd carbon. This is actually a coupled reaction: addition of a 2'-phosphate coupled to the removal of the 3'-phosphate. What does this mean? It means we bring in a new phosphate for the 2nd carbon and remove the phosphate from the 3rdcarbon; the phosphate is not moved, but replaced. If you think about it, this explains the free energy change and why it is not a spontaneous reaction in isolation.Reaction 9 involves the enzyme Enolase, which causes a dehydration of the molecule. The goal of this reaction is to induce further molecular tension in the molecule.
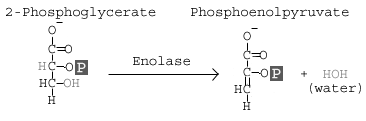 If you look at the overall reaction in the first image, you will notice that at the end of the reaction, the 2'-phosphate is shown as a High Energy Phosphate. Look at the image to the left: an -OH group from carbon 3 and an -H from carbon 2 have been removed. The removal of an H and OH (forming water) alters the electrical profile of the molecule (we took away a hydrogen bond). This isolates the phosphate group (-2 charge) near a negatively charged oxygen. The result: a high energy phosphate.
If you look at the overall reaction in the first image, you will notice that at the end of the reaction, the 2'-phosphate is shown as a High Energy Phosphate. Look at the image to the left: an -OH group from carbon 3 and an -H from carbon 2 have been removed. The removal of an H and OH (forming water) alters the electrical profile of the molecule (we took away a hydrogen bond). This isolates the phosphate group (-2 charge) near a negatively charged oxygen. The result: a high energy phosphate.The final reaction involves pyruvate kinase. If you have been paying attention to enzyme names, you may realize that the name implies that this enzyme can phosphorylate pyruvate. This is an example of historical naming: the kinase activity was discovered before the biologial implication was fully understood. International naming conventions place this pyruvate kinase in the transferase group of enzymes. Still, most books and websites use the name Pyruvate Kinase as the catalyst for this reaction.
The action of pyruvate kinase is to take the 2'-phosphate from Phosphenolpyruvate, and in a coupled reaction, add the phosphate to ADP to regenerate ATP. This is another example of substrate level phosphorylation. The product of this reaction is Pyruvate, which will have two possible fates in energy metabolism.
Aerobic Respiration: Pyruvate in Eukaryotes will go to the mitochondria and be converted to Acetyl-CoA (tomorrow's newsletter).
Anaerobic Conditions -> Fermentation: In animals, Pyruvate will be reduced by Lactate Dehydrogenase to produce Lactate.
In some fungi, fermentation takes a different route: Pyruvate is acted upon by Pyruvate Decarboxylase (removes carbon and oxygen, forming CO2) to form Acetaldehyde, which is then reduced by Alcohol Dehydrogenase to produce Ethanol.
We will talk about fermentation a little later this week.
Notes on chemical names
Many of the organic compounds that we will talk about are organic acids. For example, Pyruvate is the salt form of Pyruvic Acid. Most of the time, we will use the salt (suffix -ate) name, but remember that in the cytosol and mitochondrial matrix (eukarya) these will be in acid form. You will learn more about naming conventions in Organic Chemistry.Note: Precursor Metabolites
Remember, the intermediates of biochemical pathways can be used as building blocks for other compounds. Cells constantly have to decide what is the critical need: energy or building blocks. This is one reason there are regulatory mechanisms for proteins. The following diagram can be found at the Tumor Metabolme website presented by Drs. Erich Eigenbrodt and Sybille Mazurek from Justus Liebig University. It is an excellent reference for what can be made from the intermediates of glycolysis.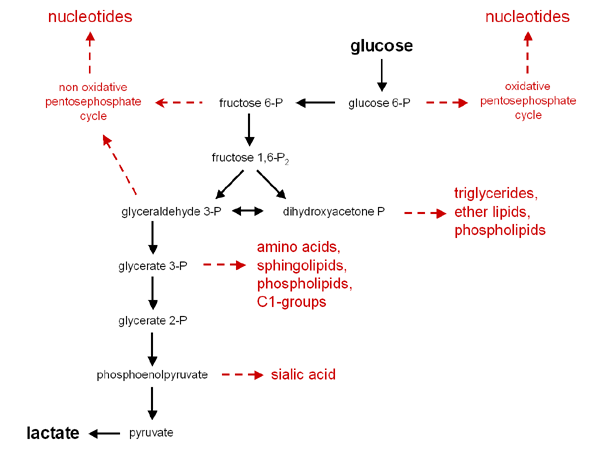
No comments:
Post a Comment